MedTech innovation in 2025 is at a crossroads. Technological breakthroughs are accelerating, yet bootstrapped founders face tighter funding and mounting pressure to deliver. Our world has shifted from the lack of viable ideas to how to execute them without raising endless funds.

According to Deloitte’s 2025 Global Healthcare Executive Outlook, 89% of life sciences leaders cite innovation as a top strategic focus. However, over 50% report achieving cost optimization as a critical barrier.
The challenge is clear: how do you build future-shaping medical technologies without the capital resources of established players?
To answer this critical question, we explore four top MedTech trends shaping the medical technology space in the second half of 2025.
Beyond trends, we also provide practical ways to build one as a bootstrapped MedTech founder or early-stage startup without sacrificing clinical relevance. You’ll also find examples of MedTech companies that have done this successfully.
Want to move faster? Book a strategy call.
Top MedTech trends in 2025
These are four trends you can build into actual products on a startup budget, without compromising on quality or compliance.
1. AI & ML-powered diagnostics
AI and machine learning play increasingly central roles in MedTech innovation in 2025 especially in diagnostic applications. From radiology to early-stage oncology detection, machine learning models are being trained on large, real-world clinical datasets to improve accuracy, reduce interpretation time, and support earlier intervention.
Artificial intelligence frameworks also automate clinical decision-making, triage workflows, and data analysis in high-volume settings.
According to J.P. Morgan’s Q1 2025 Medtech Market Report, AI-enabled diagnostic startups attracted a significant share of the $3.7 billion invested across 117 MedTech funding rounds in early 2025.
This suggests that capital continues to flow toward scalable, data-driven healthcare models that promise better outcomes and operational efficiency.
What you can build:
- A lightweight diagnostic assistant for imaging clinics
- An AI-based symptom triage chatbot for remote consultations
- A decision-support tool for specialists using real-world evidence datasets
Execution roadmap:
- Tech stack: Python, TensorFlow, FastAPI, integrated with cloud-native data pipelines
- Team strategy: Use offshore ML engineers with experience in clinical annotation and med data
- Compliance lens: Secure early guidance on HIPAA and MDR considerations from consultants or advisory boards
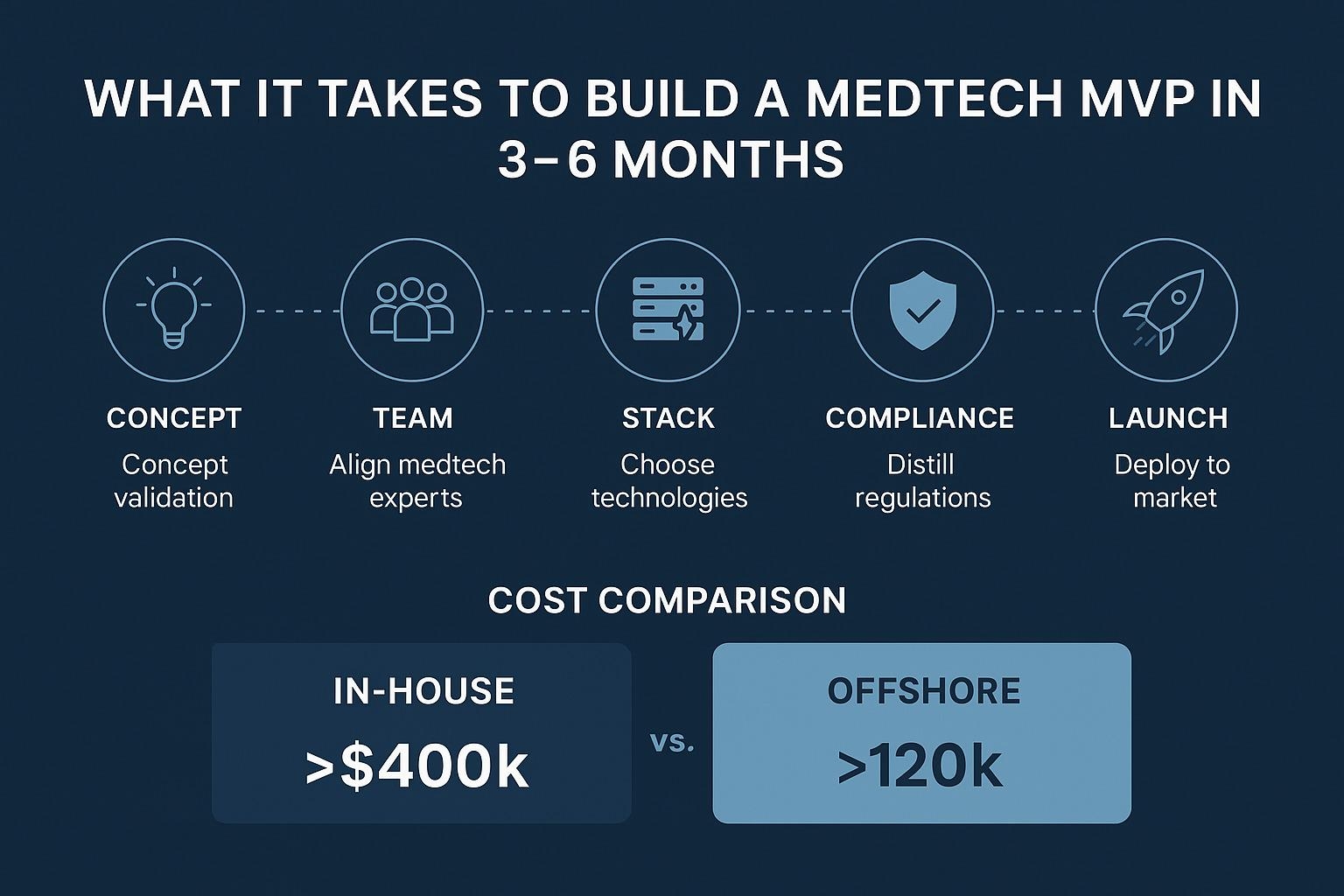
- Estimated timeline: 3–4 months to MVP, depending on data readiness and regulatory clarity
- Cost insight: U.S.-based AI dev teams (3 -4 MedTech talents) often run $350–500k/year.
Offshore staff augmentation for MedTech can reduce this to under $120k with equivalent technical scope, especially when working with pre-vetted teams trained in healthcare data handling.
Startup example: NeuralCure AI started as a lean team building an ML-powered diagnostic and simulation engine using digital twin models for clinical decision support.
Their ability to launch a narrow MVP, iterate on medical datasets, and expand through strategic imaging partnerships is a good example of how a bootstrapping founder can scale with precision.
Looking to build your diagnostic MVP? Start with a free strategy consultation.
2. Wearable biosensors
Wearable biosensors are influencing how clinicians and startups approach patient monitoring and chronic care in MedTech innovation. From glucose tracking to cardiac metrics, these devices gather continuous health data that supports outpatient care and real-time decision-making.
According to J.P. Morgan, larger MedTech venture rounds in Q1 2025 went to companies advancing real-time monitoring and digital therapeutics. Both rely heavily on wearable biosensors.
As a MedTech founder, your opportunity lies in building narrowly focused, compliant
devices designed for long-term data reliability and speed to market.
What you can build:
- A patch-based wearable for continuous glucose monitoring
- A wrist-worn biosensor to track cardiac rhythm irregularities
- A motion sensor for post-operative rehab and fall risk detection
Execution roadmap:
- Tech stack: Embedded C, Bluetooth Low Energy, cloud sync, React Native for mobile UI
- Team strategy: Offshore embedded developers for hardware-software integration, local QA for device testing
- Compliance lens: Align with ISO 13485 design controls and IEC 60601 safety standards where applicable
- Estimated timeline: 4–6 months to MVP depending on prototyping speed and device class
- Cost insight: U.S.-based embedded devs average $180/hour.
Offshore staff augmentation for MedTech with vetted engineers can reduce that significantly. Most founders see total project savings of 60 to 70 percent.
Startup example: Inviza Health built a self-charging, in-shoe wearable patch that monitors vitals and gait in home-care and military settings. They combined embedded ML with cloud analytics in a seed-stage model. It shows how founders can bring wearable biosensors to market using lean teams and external regulatory support.
Planning to launch a wearable device? Unlock free 40 hours of work.
3. Remote patient monitoring (IoMT)
Remote patient monitoring (IoMT) continues to expand across chronic care, post-surgical recovery, and clinical trials. More than 50 million Americans now use RPM technologies, and the U.S. market is expected to double by 2030, reaching close to $29 billion.
Founders are drawn to RPM because it reduces hospital load, lowers readmission rates, and extends care access without adding overhead.
What you can build:
- A home-based vitals monitoring kit (e.g., BP, SpO2, weight scale)
- A connected inhaler or spirometer that syncs with a mobile dashboard
- A remote surgical recovery tracker with alerts for complications
Execution roadmap:
- Tech stack: MQTT or HTTPS APIs, AWS IoT Core, BLE modules, edge analytics
- Team strategy: Offshore backend and firmware engineers, onshore compliance testing
- Compliance lens: HIPAA, GDPR for data; IEC 62304 for software; IEC 60601 for physical hardware
- Estimated timeline: 5–6 months depending on hardware maturity and regulatory path
- Cost insight: Founders using offshore staff augmentation for MedTech typically save 50–65% compared to in-house development, especially for IoT-heavy builds
Startup example: Peerbridge Health recently advanced its Cor MDx wearable ECG patch. The system provides real-time arrhythmia monitoring with AI-supported insights and was validated through multiple feasibility trials. Peerbridge shows how early-stage teams can take a lean but compliant path from prototype to FDA approval.
Building for RPM? Book a free strategy consultation and get feedback on your IoMT roadmap.
4. Personalized therapeutics
Personalized therapeutics are gaining traction as care models shift from population-level interventions to patient-specific protocols. Unlike biologics or gene therapies, many personalized tools in MedTech innovation 2025 are software-based.
They adapt treatment plans using biometric inputs, patient-reported outcomes, and real-time behavior tracking.
What you can build:
- A digital therapeutic that adjusts behavioral interventions based on biometric feedback
- A chronic care coaching platform with patient-specific dosage alerts
- A triage algorithm that stratifies care plans based on symptom or device data
Execution roadmap:
- Tech stack: Python, R, cloud analytics platforms, EHR APIs
- Team strategy: Offshore data scientists with digital health experience, paired with onshore clinical reviewers
- Compliance lens: HIPAA, GDPR for data privacy; GxP if used in a regulated therapeutic setting
- Estimated timeline: 6 months to MVP, contingent on model complexity and integration with EHR systems
- Cost insight: Using offshore staff augmentation for MedTech, founders can cut development spend by up to 60%, especially when leveraging reusable analytic frameworks
Startup example: Starling Medical (Seed stage) is developing UrinDx—a connected urinary health device that tracks infections and hydration in real-time. It’s a personalized therapeutic tool that blends wearables, data science, and AI-supported care, demonstrating what’s possible even at an early funding stage.
Need help planning a digital therapeutic? Book your free strategy call.
What to do next if you’re building MedTech on a lean budget
MedTech innovation is ripe with opportunity, even for founders navigating lean budgets. From AI-powered diagnostics to wearable biosensors, remote patient monitoring, and personalized therapeutics, the path forward doesn’t require building from scratch or raising a Series A.
If you’re a bootstrapping or early-stage MedTech founder, execution is your edge. With our Silicon Valley-grade experts and access to the top 1% offshore talent, we help you build regulatory-ready devices on time and under budget.
Whether you’re developing your first MVP or scaling toward FDA clearance, we align world-class engineers and domain-aligned teams to move faster without compromise.
Ready to move? Book your strategy session or claim free development hours to get started.


